Understanding the differences between synthethic NKP fertilisers and Organic fertilisers
Main conditions |
synthethic |
etefert |
| NPK | ✔✔ | ✔ |
| Micronutrients (trace elements) | ✖ | ✔ |
| Organic matter | ✖ | ✔ |
| Humic, Fulvic acids | ✖ | ✔ |
| Effective availabillity of nutrients | ✔ | ✔✔ |
| Optimal water absorption of the soil | ✖ | ✔ |
| Optimal aeration of the soil | ✖ | ✔ |
| Optimal nutrient ratio (availlable analysis) | ✔✔ | ✔ |
| Healthier plants, resistent to pets and diseases | ✖ | ✔ |
| Environment friendly | ✖ | ✔ |
| Substainable product | ✖ | ✔ |
| Easy to use and storage | ✔ | ✔ |
| Crop quality increase | ✔ | ✔✔ |
| Yield increase | ✔ | ✔ |
|
Price total per package (NPK, micronutrients,OM, humic acids, sustainabillity) |
✔✔ | ✔✔✔ |
Main benefits of using ETE.FERTTM organic fertiliser
1) Plant Growth
ETE.FERTTM Organic fertilizers provide nutrients necessary for plant growth, with the benefit of being slower-acting and gentler than chemical fertilizers, so that you are less likely to overfeed and chemically burn your plants. Organic fertilizers are not in a form that is immediately absorbed by plants, but rather must be first broken down by soil bacteria and fungi into forms that plants can absorb. This means that, unlike chemical fertilizers, organic fertilizers are not easily washed away in a heavy rainstorm or irrigation session, and that the plants get the benefit of nutrients for growth more evenly over a longer period of time rather than all at once.
3) Slow Release of Nutrients
When fertilizers are mixed into the soil, the nutrients are absorbed from the soil by the roots of the plant. In synthetic fertilizers, these nutrients are in ready to use form and when mixed into the soil, can be immediately absorbed by the roots and hence, the plant. There is however a real danger that the roots absorb more nutrients than necessary, causing the roots and plant to burn up. On the other hand, ETE.FERTTM organic fertilizers do not contain nutrients in an easily usable form. When they are mixed into the soil, the microorganisms like bacteria that are in the soil, have to work on the fertilizer, break it up and release the nutrients. This is a slow process and so there is no danger that too many nutrients are ever available to the plant. As such there is low chance for a ‘plant burn’ when organic fertilizers are used.
5) Long-term Benefits to the Environment
Synthetic fertilizers also tend to release many chemicals into the soil that contain nutrients helpful to soil but may also contain elements that are not easily biodegradable. These may go on to contaminate our lands and our water. On the other hand, by definition, ETE.FERTTM organic fertilizers almost always have only biodegradable contents.
2) Soil Improvement
ETE.FERTTM Organic fertilizers help improve soil structure and nutrient content over time. While chemical fertilizers simply add water-soluble chemicals which are either absorbed by the plant roots or leach away, potentially polluting water resources, organic fertilizers add organic matter that helps the soil to retain moisture and nutrients. Sandy soils in particular can benefit from the addition of organic fertilizers
4) Long-term Benefits to the Soil
Chemical fertilizers are manufactured with the sole purpose of helping the growth of a plant. As a result while they may contain a better balance of all the major nutrients that a plant needs, they also contain certain harmful elements that can cause acidity in the soil. This can kill the helpful microbes that live in the soil and studies indicate that long-term use of chemical fertilizers can cause great damage to the soil. On the other hand, since ETE.FERTTM organic fertilizers need these microbes to work on them to release the nutrients, they end up stimulating the growth of these microorganisms, ensuring long-term fertility of the soil.
What you should know about plant nutrients
Making your crop thrive will need more than just NPK. In fact there are about 17 essential plant nutrients that must be available for your crops to thrive. And because The Law Of The Minimum applies, crop yields will be compromised even if just one or more of these 17 nutrients lack in the soil no matter if all the other s are sufficiently available.
| 1. MacroNutrients | N, P and K |
| 2. Secondaary nutrients | Mg, S and Ca |
| 3. Micronutrients | B, Cl, Mn, Fe, Ni, Cu, Zn, Mo |
| 4. Other crucial elements | H, C and O |
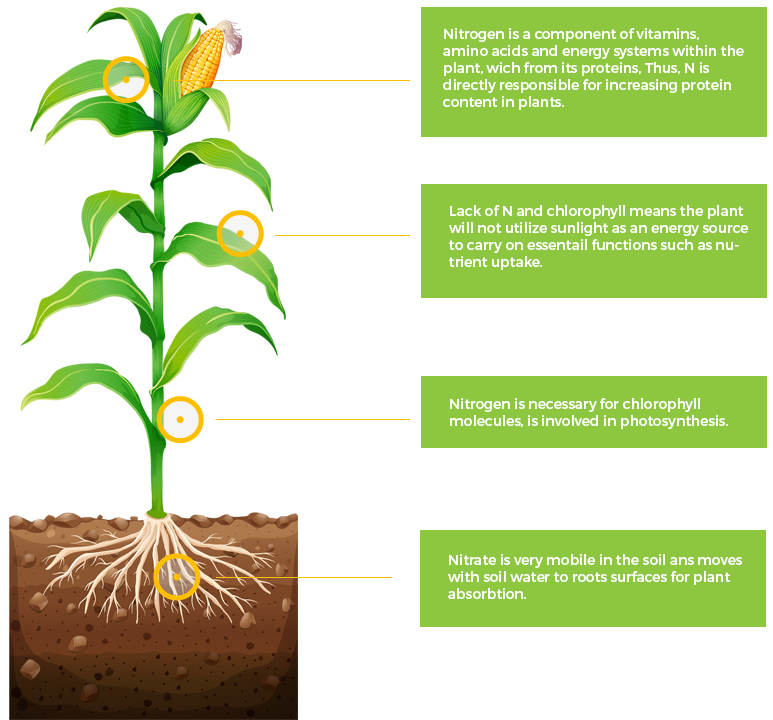
N
Nitrogen
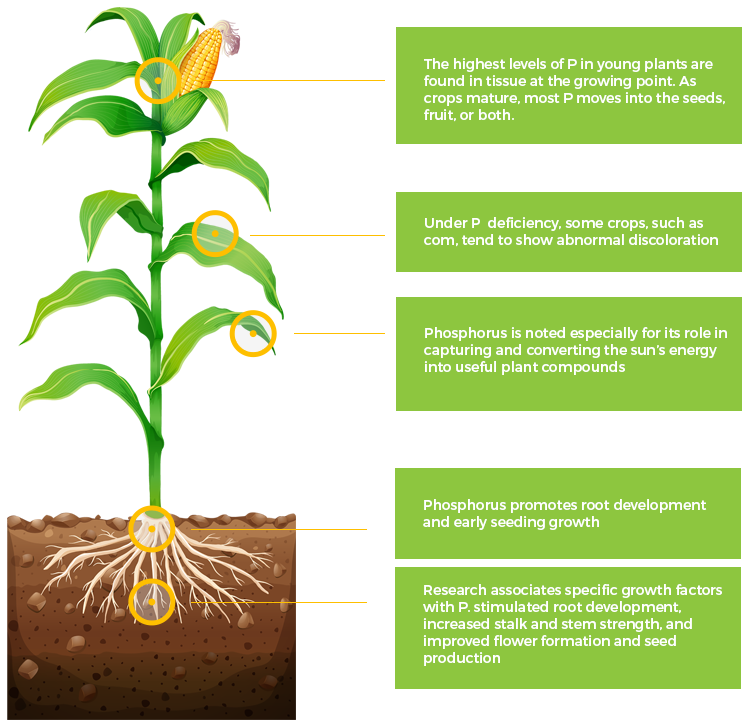
P
Phosphorus
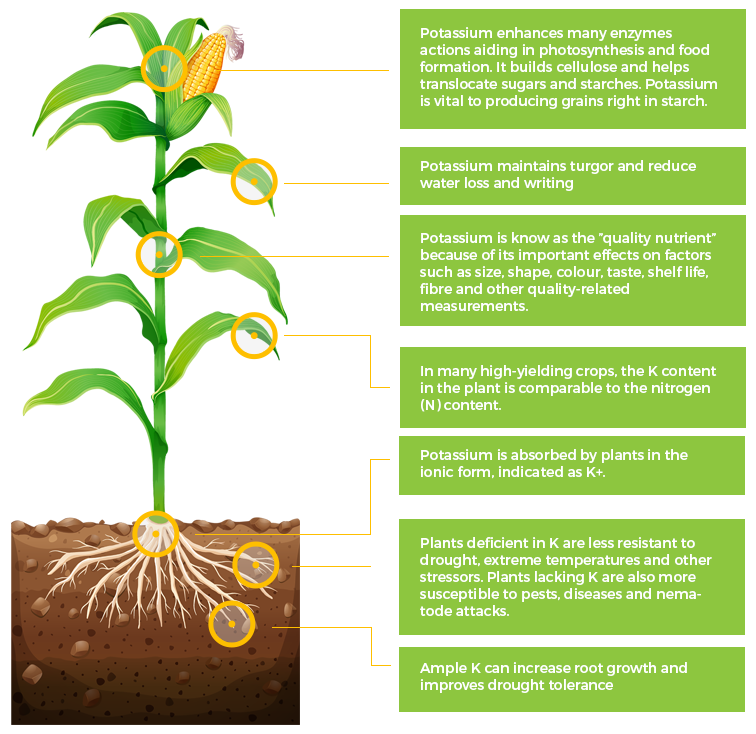
K
Potassium
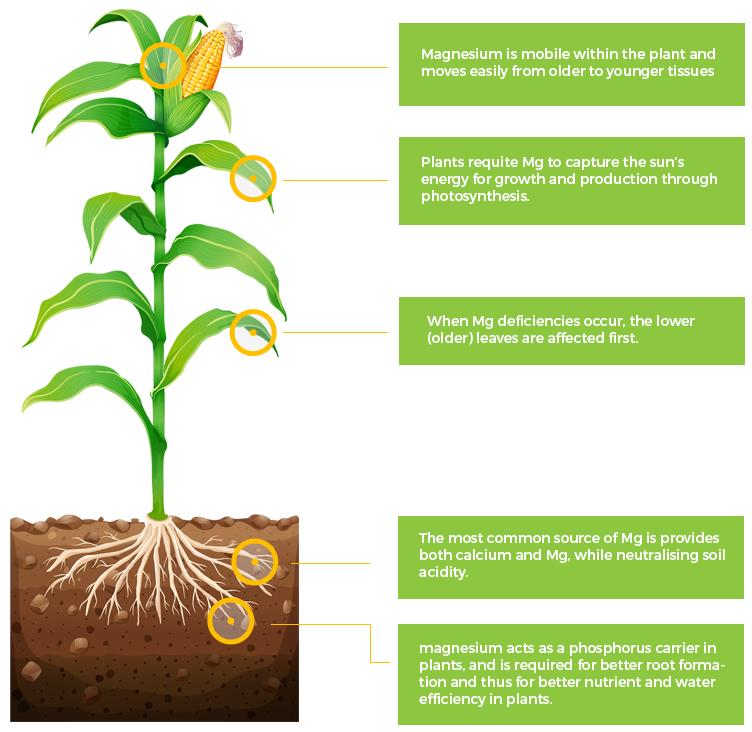
Mg
Magnesium
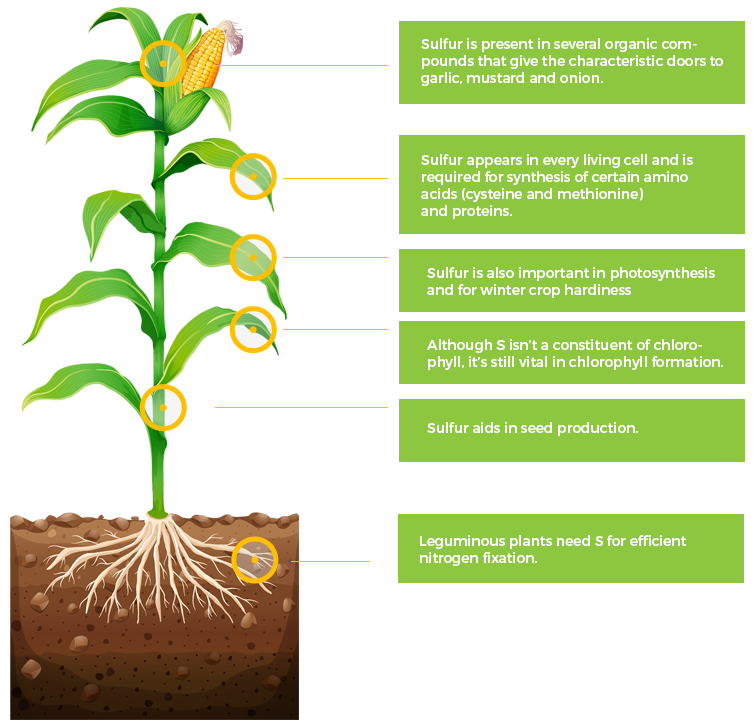
S
Sulfur
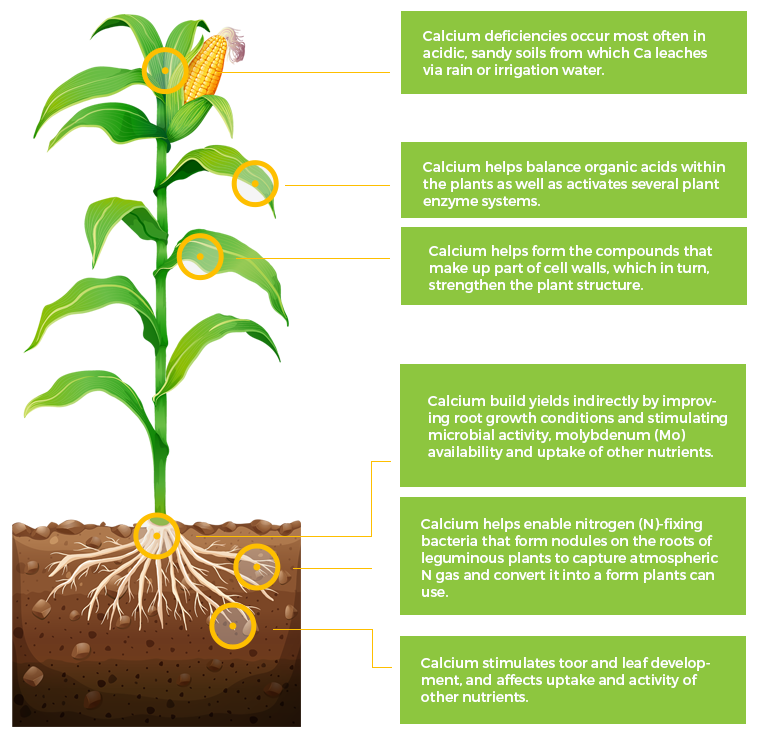
Ca
Calcium
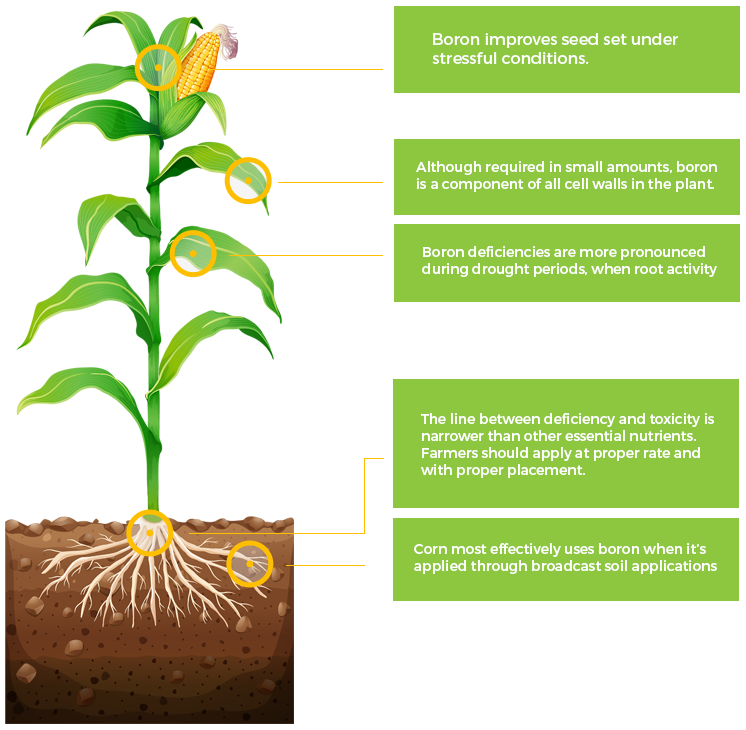
B
Boron
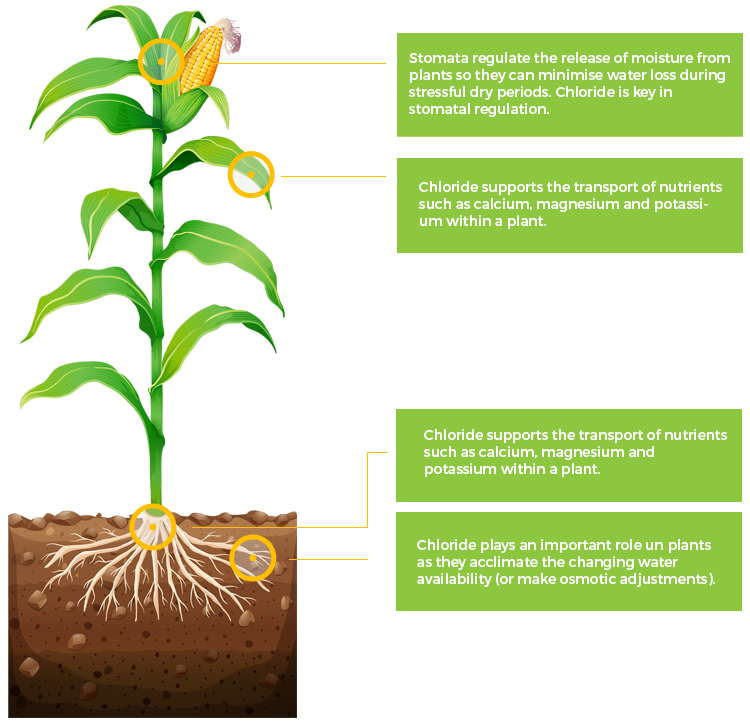
Cl
Chlorine
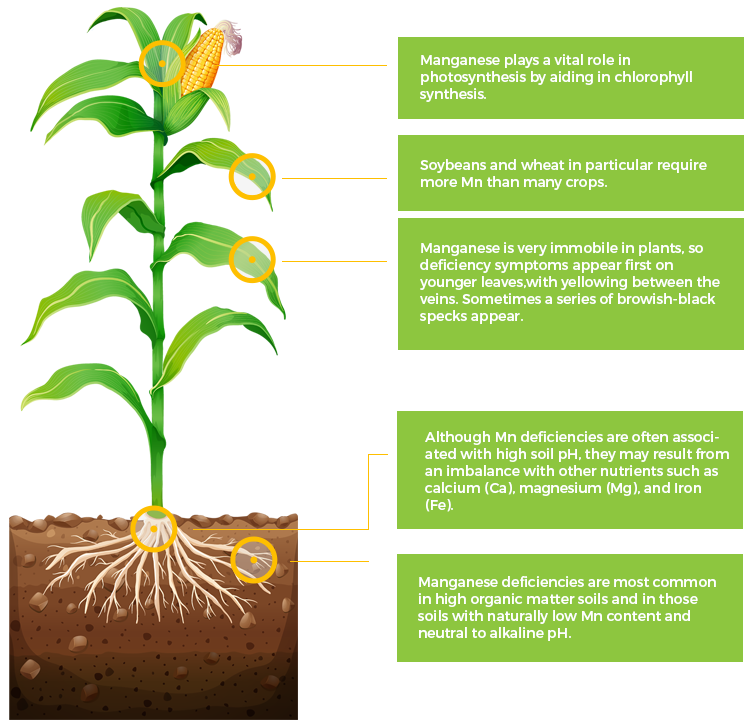
Mn
Manganese
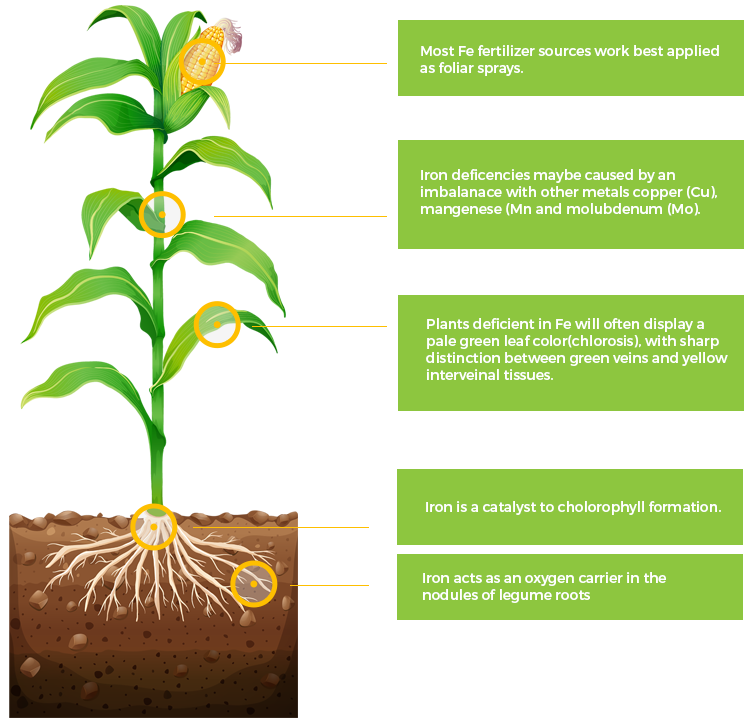
Fe
Iron
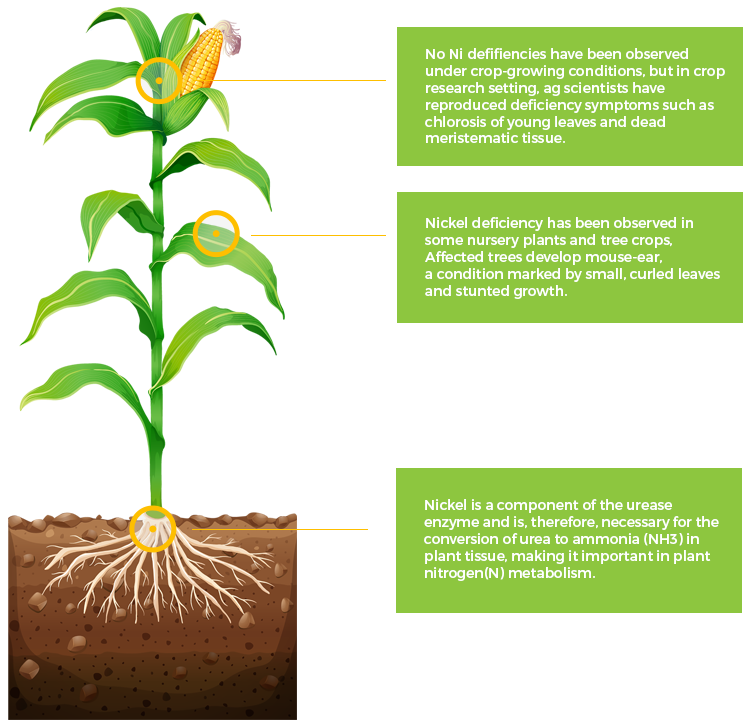
Ni
Nickel
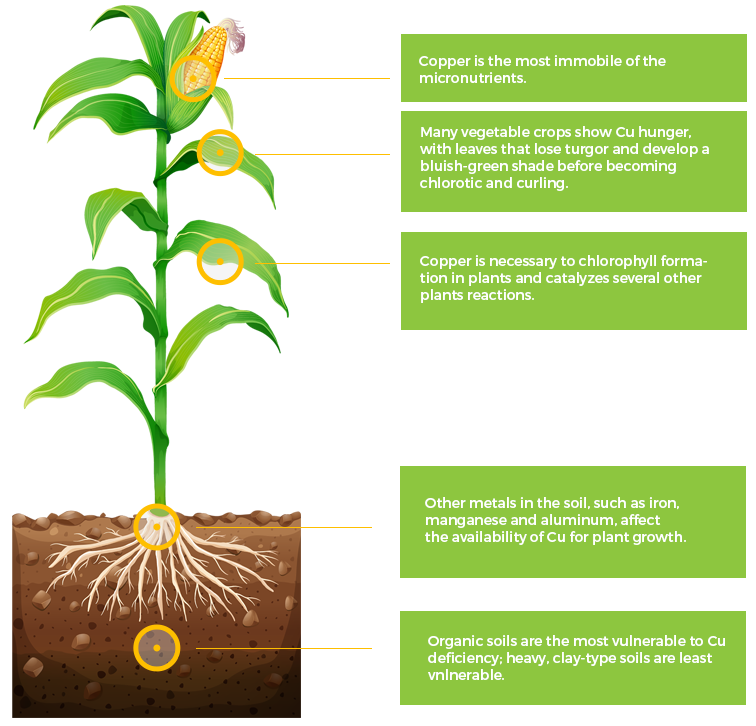
Cu
Copper
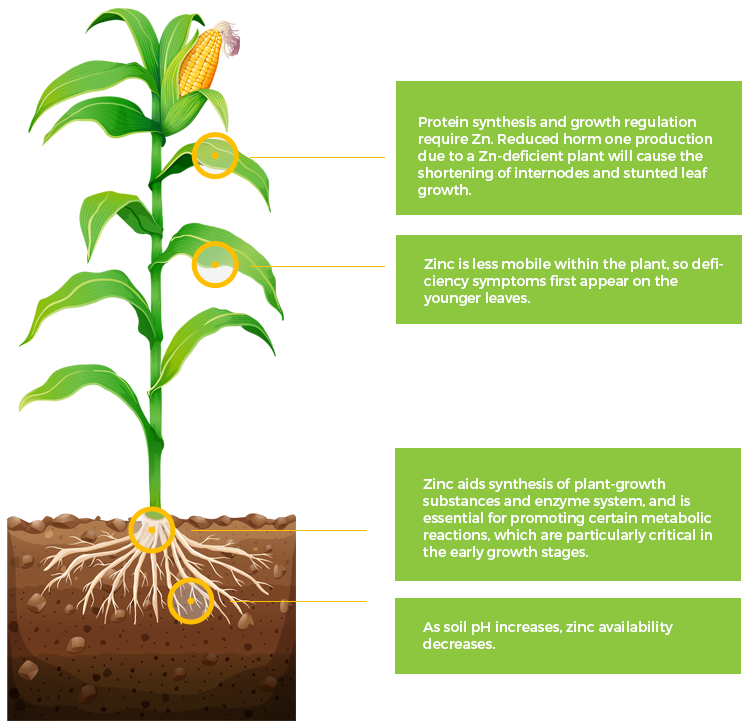
Zn
Zinc
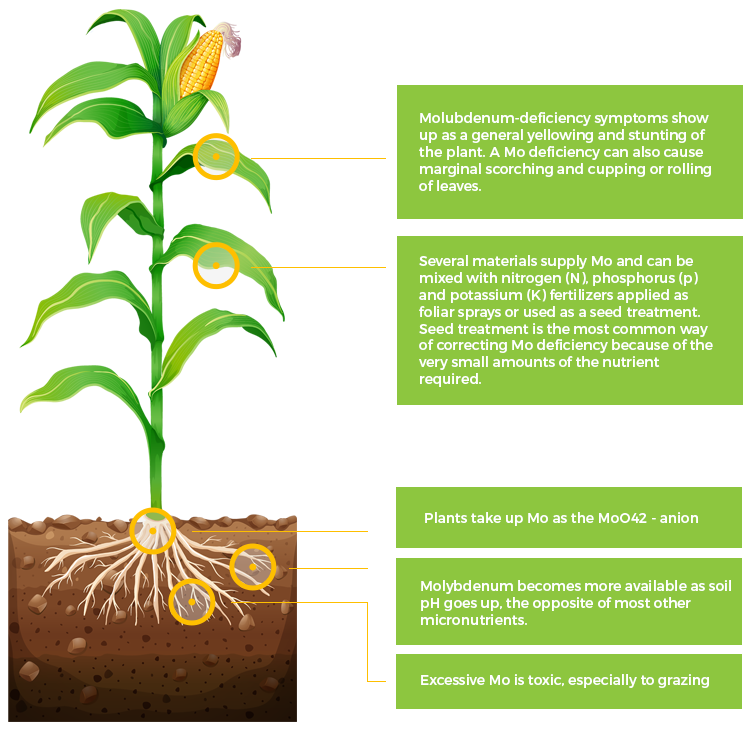
Mo
Molybdenum
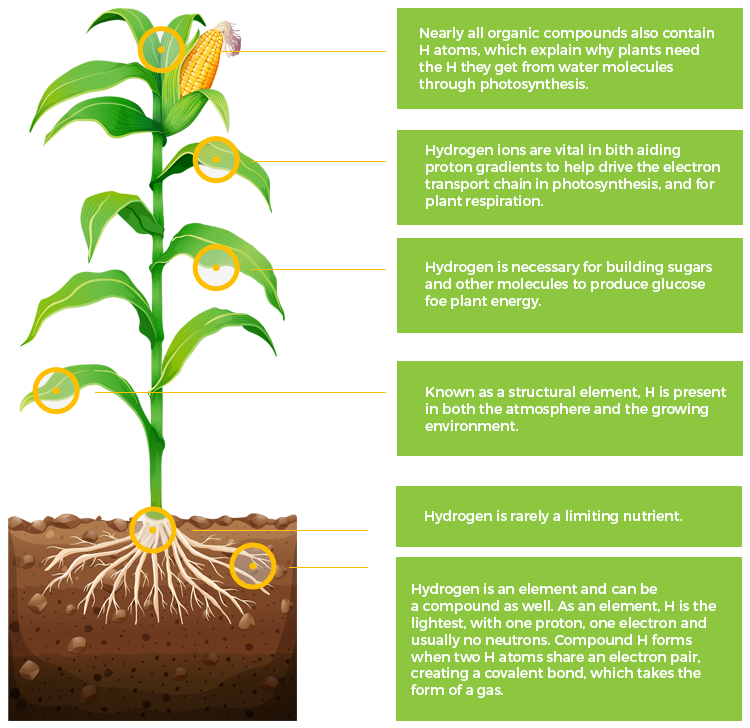
H
Hydrogen
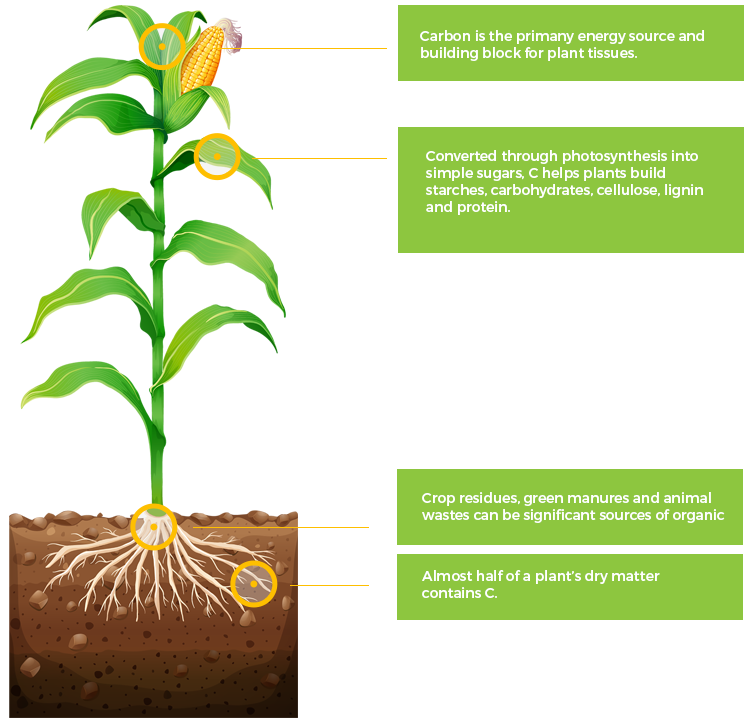
C
Carbon
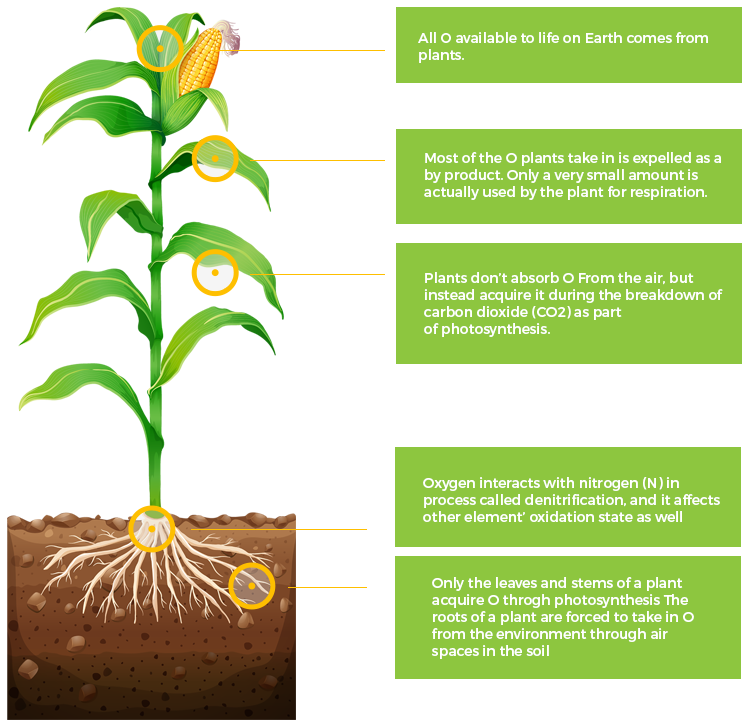
O
Oxygen
nitrogen
phosphorus
potassium
magnesium
sulfur
calcium
boron
chlorine
manganese
iron
nickel
copper
zinc
molybdenum
hydrogen
carbon
oxygen
3. Loss of soil organic matter
Topsoil 25 cm, surface 10.00 m2
Gravity 1300 kg/m3, 2% o.m. = 65.000 kg o.m.
Cultivation soil = 65.00 kg o.m.
Naturally yearly breakdown 1,95 ton / ha / y
Crop residues in the field 750 - 1250 kg o.m.
Replacement of organic matter 700 - 1200 kg o.m. = 1100 - 1850 kg Organic Fertilizers